VANDA Pharmaceuticals recently announces FDA grants Orphan Drug Designation for VGT-1849B, a novel and selective candidate for the treatment of Polycythemia Vera
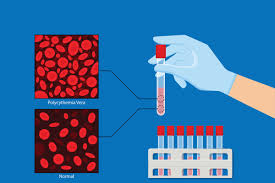
WASHINGTON — PRNewswire — Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) on Aug. 28, announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation for VGT-1849B, a selective peptide nucleic acid-based JAK2 inhibitor for the treatment of polycythemia vera (PV).
PV is a chronic myeloproliferative disorder characterized by aberrant hematopoiesis of myeloid lineage with exuberant red cell production and increased release of pro-inflammatory cytokines. More than 95% of PV patients harbor the JAK2 V617F gain-of-function mutation leading to aberrant JAK2 production.1 The prevalence of PV in the United Statesis estimated to affect 44 to 57 per 100,000 people.2
VGT-1849B is an antisense oligonucleotide (ASO) that utilizes a novel backbone chemistry, OliPass Peptide Nucleic Acid (OPNA), that has been derived from peptide nucleic acid (PNA) by rationally introducing cationic lipid moieties onto nucleobases. By covalently attaching cationic lipid groups onto PNA, cell permeability and affinity for RNA are markedly improved.
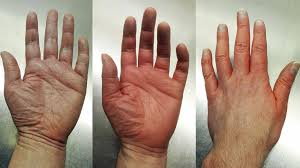
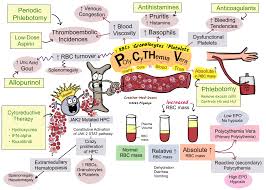

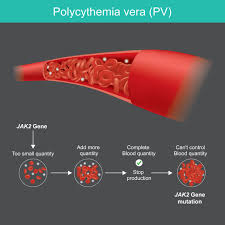
By selectively targeting JAK2 mRNA, VGT-1849B reduces downstream signaling and JAK2V617F-driven autonomous cell proliferation. VGT-1849B targets JAK2 with high precision and effectively reduces JAK2 protein production, without any off-target kinase effects. Inhibiting JAK2 acts to suppress hematopoiesis, consequently reducing red blood cell, neutrophil, platelet, and lymphocyte production. The ability of VGT-1849B to reduce JAK2 protein may alleviate the disease burden that patients with PV face with a favorable safety profile, resulting in a higher quality of life for patients.
VANDA-CS@collectedstrategies.com
Source: Vanda Pharmaceuticals Inc.