VIRUS TODAY: Vaccine gains momentum, NFL season in chaos
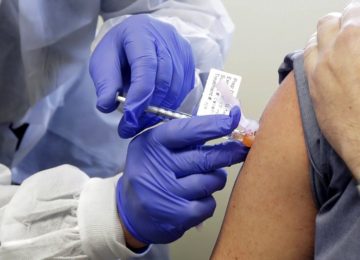
FILE – In this March 16, 2020, file photo, Neal Browning receives a shot in the first-stage safety study of a potential vaccine for COVID-19 at the Kaiser Permanente Washington Health Research Institute in Seattle. Moderna Inc., said Monday, Nov. 16, its