Dwayne Johnson debuts ‘Young Rock’ trailer on Instagram: ‘Every hero has an origin story’
Dwayne “The Rock” Johnson is returning to the small screen with the help of NBC. — FOX News

Dwayne “The Rock” Johnson is returning to the small screen with the help of NBC. — FOX News

FILE – In this March 4, 2020, file photo Chairman of the Joint Chiefs of Staff Gen. Mark Milley testifies to Senate Armed Services Committee about the budget on Capitol Hill in Washington. President-elect Joe Biden will inherit Milley as his senior

This satellite photo combo provided Sunday Jan. 17, 2021 by Planet Labs, Inc. shows the destruction of U.N. World Food Program warehouses at the Shimelba refugee camp in Ethiopia’s Tigray region on Jan. 5, 2021, bottom center left, and before it was

Supporters of leading opposition challenger Bobi Wine cheer as election officials count the ballots after polls closed in Kampala, Uganda, Thursday, Jan. 14, 2021. Ugandans voted in a presidential election tainted by widespread violence that some fear could escalate as security forces
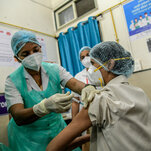
The campaign is unfolding in India, a country that has reported more than 10 million coronavirus infections. — NYT: Top Stories

FILE – In this Dec. 23, 2020 file photo, a woman participates in a protest against Brazilian President Jair Bolsonaro’s handling of the new coronavirus pandemic in Brazilia, Brazil. The country hasn’t approved a single vaccine yet, and independent health experts who
First HER2 directed medicine approved for patients with gastric cancer in a decade TOKYO & MUNICH & BASKING RIDGE, N.J.–(BUSINESS WIRE)–Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) and AstraZeneca’s ENHERTU® (fam-trastuzumab deruxtecan-nxki) has been approved in the U.S. for the treatment of

Friday: The New York State’s vaccine rollout is not going smoothly. — NYT: Top Stories

A bus in New York City which careened off a road in the Bronx neighborhood of New York is left dangling from an overpass Friday, Jan. 15, 2021, after a crash late Thursday that left the driver in serious condition, police said.